C+K Probe-Sanitizing-Set

Skin measurements in difficult times
In times of social distancing and hygiene rules during the COVID-19 pandemic, skin measurements have become more difficult and time consuming. However, the tests need to go on. So we have put a lot of thorough researching and testing into a C+K probe/devices sanitizing set and a corresponding protocol. The aim is to allow you to use the C+K instruments in the best possible way.
Zanitize, a hand disinfectant developed by the renowned German Fraunhofer Institute, was especially chosen because of its skin-friendliness. With 76 % of ethanol it is also very well suited to sanitize surfaces (e.g. most C+K probes and devices). Zanitize is approved as a biocidal product according to the Biocide Regulation (VO [EU] No. 528/2012) for hygienic hand disinfection with the BAuA approval number N-89415. According to the manufacturer it has limited virucidal properties. Effectiveness: Sars-Cov-2 (Corona or Covid-19) Bactericidal, levurocidal, fungicidal, tuberculocidal, mycobactericidal, limited virucidal according to RKI (HBV/HIV, HCV, BVDV, Vaccinia Viruses), Adenovirus, Poliovirus, Rotavirus and Norovirus.
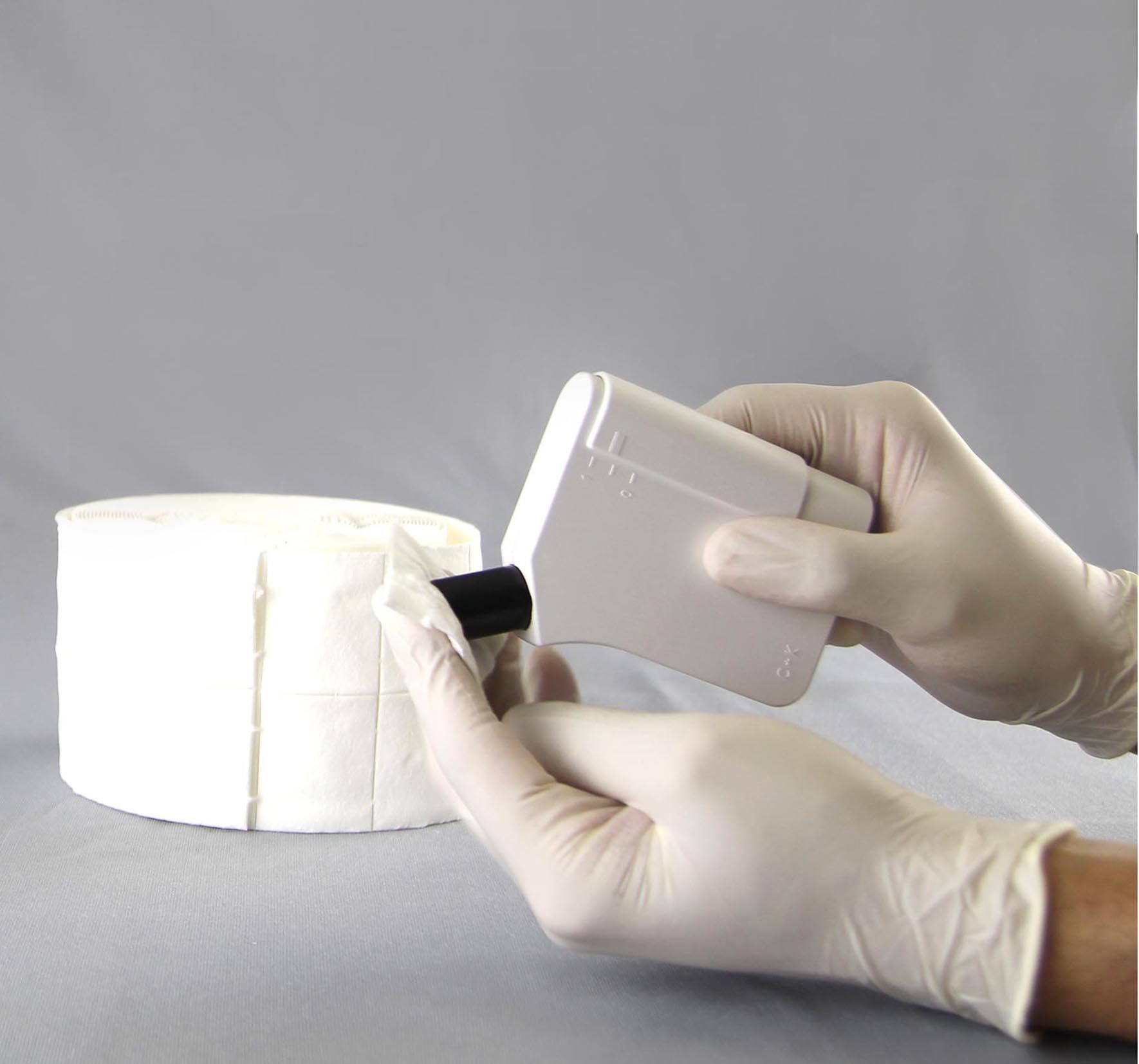
We have worked on a cleaning protocol for most C+K probes and devices. The described procedures were tested at C+K to be compatible with the material of the probes/devices and when dried completely to not bear any influence on the measurement results.
Zanitize, a hand disinfectant developed by the renowned German Fraunhofer Institute, was especially chosen because of its skin-friendliness. With 76 % of ethanol it is also very well suited to sanitize surfaces (e.g. most C+K probes and devices). Zanitize is approved as a biocidal product according to the Biocide Regulation (VO [EU] No. 528/2012) for hygienic hand disinfection with the BAuA approval number N-89415. According to the manufacturer it has limited virucidal properties. Effectiveness: Sars-Cov-2 (Corona or Covid-19) Bactericidal, levurocidal, fungicidal, tuberculocidal, mycobactericidal, limited virucidal according to RKI (HBV/HIV, HCV, BVDV, Vaccinia Viruses), Adenovirus, Poliovirus, Rotavirus and Norovirus.
We have worked on a cleaning protocol for most C+K probes and devices. The described procedures were tested at C+K to be compatible with the material of the probes/devices and when dried completely to not bear any influence on the measurement results.